Multiwfn forum
Multiwfn official website: //www.umsyar.com/multiwfn. Multiwfn forum in Chinese: http://bbs.keinsci.com/wfn
You are not logged in.
- Topics:Active|Unanswered
#12021-04-11 20:20:01
- Pratik7320
- Member
- Registered: 2021-01-28
- Posts: 11
Mayer-bond-order-analysis
This is Mayer bond order analysis
This is bond path image of bal2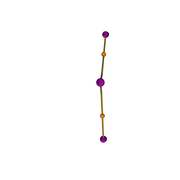
Now, my question is if the mayer bond order between 2(Al) & 3(Al) =1.087242( that is, it has single bond character), then why this bond order is not reflected in the bal2-bondpath.png between 2(Al) & 3(Al)? (it seems like there is no bond between 2(Al) & 3(Al))
So, I have attached my input file.
my input file is as follows:-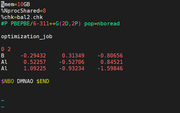
Please reply sir.
Offline
#22021-05-05 02:41:11
Re: Mayer-bond-order-analysis
Please never use diffuse functions when calculating Mayer bond order, this very important point has been mentioned in Section 3.11.1. Diffuse functions will break physical meaning of calculated Mayer bond order.
If the bond order of Al-Al is still large after removing diffuse functions, there may exist three-center bond, the electron sharing is evident even though the two Al atoms are not spatially close with each other.
Offline