Multiwfn forum
Multiwfn official website: //www.umsyar.com/multiwfn. Multiwfn forum in Chinese: http://bbs.keinsci.com/wfn
You are not logged in.
- Topics:Active|Unanswered
#12021-01-28 01:43:46
- Quark
- Member
- Registered: 2020-06-24
- Posts: 15
Calculation of the energy of the halogen bond
Dear Professor Tian Lu,
In my study, I need to calculate the energy of the halogen bond Cl-O. (Figure1). To do this, I used the SAPT method. According to calculations, the energy of interaction between fragments is estimated at -4.4 kcal/mol. The results of energy decomposition show that long-range interactions (electrostatic and dispersion attraction interactions) prevail. In order to calculate the halogen bond energy, a model calculation was made where halogen atoms were replaced by hydrogen atoms, thus the distances between oxygen and hydrogen atoms are greater than the Van der Waals radius.(Figure3) Thus, the energy between the model fragments was -2.5 kcal/mol. Is it true that the energy of the halogen bond is the difference between the energy of the fragments with the halogen bond and the model system?
(-4.4 + 2.5) / 2 = -0.95 kcal/mol the energy of the halogen bond Cl-O.
Can it be said that the dimer is mainly retained by halogen bonds Cl-O and long-range interactions?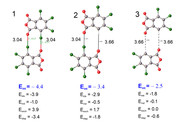
Thank you in advance for your answers.
--
Best regards
Eugene
Last edited by Quark (2021-01-28 01:51:14)
Offline
#22021-01-28 11:56:17
Re: Calculation of the energy of the halogen bond
Since the two molecules are binded together by two identical halogen bonds, and there is no other sources noticeably contributing to the complexation, in my opinion it is best to estimate E_int of each halogen bond as -4.4/2 = -2.2 kcal/mol.
Offline
#32021-01-30 00:20:10
- Quark
- Member
- Registered: 2020-06-24
- Posts: 15
Re: Calculation of the energy of the halogen bond
Dear Professor Tian Lu,
Thank you for dispelling doubts
--
Best regards
Eugene
Offline