Multiwfn forum
Multiwfn official website: //www.umsyar.com/multiwfn. Multiwfn forum in Chinese: http://bbs.keinsci.com/wfn
You are not logged in.
- Topics:Active|Unanswered
#12020-09-24 03:19:53
- shenaya
- Member
- Registered: 2019-10-16
- Posts: 34
Laplacian electron density of energertic materials
Dear All,
I'm performing a study on the impact sensitivity of the energetic materials using AIM analysis. Here I'm using zwitterionic salts and to optimize the molecules I've used IEFPCM=water as the solvent. Gas phase optimizations are not successful as the H atom tend to dissociate and attache to O or N
CAM-B3LYP/6-31g(d)/IEFPCM=water
However I can not observe the desired trend from the laplace of electron density analysis.
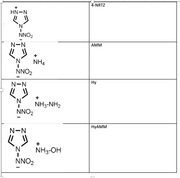
electrondensity laplace Sensitivity rank
NRTZ 0.394566243 -0.817435389 1
AMM 0.396351132 -0.80044764 3
Hy 0.386374712 -0.76654905 2
HyAm 0.38592834 -0.765011656 2
I expected highest negative value from least sensitive molecule and vise versa.
Hy and HyAm values are ok
NRTZvalue is overestimated.
Can you please comment on this.
Thanks,
Shen
Offline
#22020-09-24 03:39:50
Re: Laplacian electron density of energertic materials
Each BCP has corresponding electron density and its Laplacian, I don't know which BCP was studied.
According to my knowledge, there is seemingly no clear relationship between BCP properties and impact sensitivity. Have you ever seen any relevant literature discussing impact sensitivity in terms of BCP properties?
Online
#32020-09-24 05:51:10
- shenaya
- Member
- Registered: 2019-10-16
- Posts: 34
Re: Laplacian electron density of energertic materials
In these molecules, N-NO2 is the trigger bond. So I've obtain the bond critical point between N-NO2 and obtained the properties at BCP.
In this article "Charge Density Distribution, Electrostatic Properties, and Impact Sensitivity of the High Energetic Molecule TNB: A Theoretical Charge Density Study.
The most sensitive bond is studied using laplacisan electron density.
As per attached discussion they have identified the weakest bond using laplacian electrons density, Gr, Vr and Hr.
Offline
#42020-09-24 07:53:34
Re: Laplacian electron density of energertic materials
According to your statement, the properties at BCP of N-NO2 bond is indeed somewhat related to impact sensitivity, which, however, may be also controlled by other factors, and thus the observed impact sensitivity may not be fully explained by AIM theory.
These are solid materials, it is questionable to employ solvation model to represent surrounding environment as water. If you already have crystal structure (.cif file), you can extract a cluster (seehttps://youtu.be/fLXr6S-8-8gon how to do), fix coordinate of the boundary molecules and then only optimize the central zwitterionic salt. Due the crystal environment it is expected that H will not to dissocate. After that, you can check if the BCP properties are in line with your expectation.
Online
#52020-09-24 15:26:47
- shenaya
- Member
- Registered: 2019-10-16
- Posts: 34
Re: Laplacian electron density of energertic materials
Thank you very much for your suggestion. I will definitely try this now
Offline